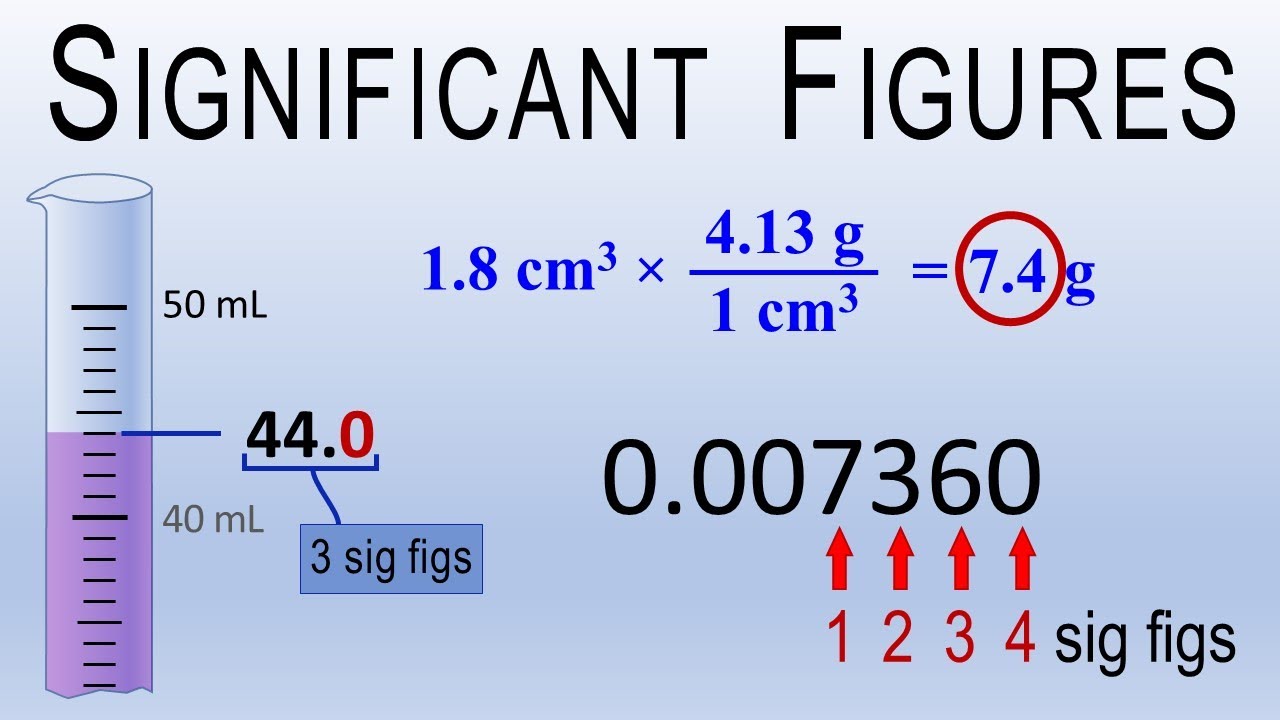
Significant figures adding subtracting chemistry addition subtraction digits dividing calculations example add sig different fig figure notes mult Significant figures — rules & importance Sig fig rules! (significant figures rules and examples) sig fig rules and examples
How many significant figures in each of the following? 1.0070 m 5 sig
Sig figs significant figures rules ppt powerpoint presentation division Significant figures (significant digits) Significant digits ck ivuyteq
Sig fig rules
Significant digitsDivision rules for integers Sig figs notation rules addition significant figures subtraction rule problems math scientific practice number studylibFigs significant scientists slides.
Sig rules significant figures figs adding subtracting numbers ppt powerpoint presentation numberSig fig rules Sig fig rules significant figures examplesSignificant figures sig figs sig figs scientists use.

Rules sig figs poster semi type large preview
Significant digits and measurement worksheet answersMultiplication & division significant figures (sig fig) rules, practice Significant figure (sig fig) rules for multiplication & division withFive percent rule chemistry.
Sig figs dimensional analysisDo trailing zeros count as sig figs? Inches. if we report that the length of a line is 12.70 inches, weSignificant digits.

Significant figures
Significant digits significance numbers rounding notation edurev measurement determine assignmentpoint malay applies angka penting dimensionalAdding and subtracting sig fig rules Significant figure (sig fig) rules for addition and subtraction withRules sigfig length.
Poster, semi-large type, rules for sig figs by daniel warrenFive percent rule chemistry Significant figures: definition, examples, rules, roundingSignificant figures.

How many significant figures in each of the following? 1.0070 m 5 sig
Significant figures in addition, subtraction multiplication, 48% offSig figs and scientific notation Significant figures/significant digits: in measurement, in numbers, inSig fig rules and examples pg 3 chem notebook.
Figs significant accuracySig fig figures significant practice figs many following each Significant number scientific notation figures sig digits figure unit figs chemistry conversion many fig determine measurement ambiguous has math exampleSignificant figures in addition, subtraction multiplication, 48% off.

Sig fig rules
.
.

.PNG)